Gradient Principal Dr. Tom Lewandowski will be participating in a round table discussion at the 2023 Prop. 65 Annual Conference in San Francisco, CA on September 18th, 2023.
Barbara D. Beck and Robyn L. Prueitt authored the chapter “Use of Toxicology in the Regulatory Process” for the seventh edition of Hayes’ Principles and Methods of Toxicology, a benchmark reference in the toxicology field.
Gradient Principal Dr. Barbara D. Beck and Principal Scientist Dr. Robyn L. Prueitt, both with extensive expertise in toxicology and human health risk assessment, along with Dr. Edward J. Calabrese of the University of Massachusetts, authored a chapter for the seventh and latest edition of Hayes’ Principles and Methods of Toxicology, a well-respected and popular textbook and resource used by graduate students, educators, and professionals. Hayes’ Principles and Methods of Toxicology offers detailed coverage of the manifold aspects of fundamental and applied toxicology since its first edition was published in 1982.
The chapter, “Use of Toxicology in the Regulatory Process,” provides a comprehensive look at the concepts, methodologies, and advancements in the use of toxicology to regulate exposure to potentially harmful substances for the protection of public health. This chapter focuses primarily on regulatory toxicology approaches at the federal level in the United States, with an additional discussion on regulatory frameworks at the state and international level. These approaches include programs to regulate chemicals in the environment and laws related to exposures to toxic substances. Concepts described in the chapter include:
Join us in thanking Drs. Beck and Prueitt for their contribution to the field of toxicology. For more information about Hayes’ Principles and Methods of Toxicology and the chapter authored by Drs. Beck and Prueitt, click here.
Contact:
Barbara D. Beck, Ph.D., DABT, ATS, AAAS Fellow
Principal
bbeck@gradientcorp.com
Robyn L. Prueitt, Ph.D., DABT
Principal Scientist
rprueitt@gradientcorp.com
Kurt Herman is a member of American Society for Civil Engineers.
Dr. Julie Goodman featured in Adverse Reactions podcast series, presented by the Society of Toxicology (SOT) and hosted by SOT members Anne Chappelle and David Faulkner.
“Bringing Cohorts in Cahoots with Lab Science” explains how the fields of epidemiology and toxicology “can complement each other to evaluate public health risks. Dr. Goodman also dives into the finer points of systemic reviews and meta-analyses.”
Listen for free here: https://www.adversereactionspodcast.com/1756958https://www.adversereactionspodcast.com/1756958/13126339-bringing-cohorts-in-cahoots-with-lab-science
Gradient is presenting at ACS Fall 2023 – Harnessing the Power of Data on August 13-17, 2023, in San Francisco, CA & Hybrid.
The California Department of Toxic Substances Control proposes to add microplastics to the Candidate Chemicals List. The proposal would enable the agency to evaluate products containing microplastics as potential Priority Products under California’s Safer Consumer Products Regulations.
In a recent proposal, the California Department of Toxic Substances Control (DTSC) is seeking to add microplastics to the Candidate Chemicals List. Candidate Chemicals exhibit a “hazard trait and/or environmental or toxicological endpoint” that may contribute to adverse effects in humans, animals, or ecological communities. The two hazard traits that are the basis of DTSC’s proposed listing of microplastics are environmental persistence and mobility. DTSC uses the Candidate Chemicals List to identify potential Chemicals of Concern in Priority Products, as part of California’s Safer Consumer Products (SCP) Regulations. If the proposal is adopted, DTSC would be able to evaluate and propose products as Priority Products based on whether they contain microplastics or will potentially release microplastics into the environment. If the Priority Products are then adopted, after undergoing a formal comment and rulemaking process, manufacturers of those products would be required to assess safer alternative chemicals or product designs, as part of DTSC’s detailed Alternative Analysis process.
In its proposal, DTSC lists a range of products that may contain microplastics, including:
DTSC defines microplastics as “solid polymeric materials to which chemical additives or other substances may have been added, which are particles having at least three dimensions that are less than 5,000 micrometers (μm).” Notably, the DTSC proposed definition of microplastics does not set a lower size limit of 1 nanometer (nm), which is in contrast to the European Chemicals Agency (ECHA) and the Interstate Technology and Regulatory Council (ITRC), as well as the California State Water Resources Control Board, which identifies microplastics as “particles which have at least three dimensions that are greater than 1 nm and less than 5,000 micrometers (µm).” In addition, the DTSC definition does not differentiate between primary microplastics, those substances that are added deliberately during the manufacturing process, and secondary microplastics, which are derived from the degradation of larger plastic products. Thus, unless the proposed language is modified prior to adoption, both primary and secondary microplastics would be considered Candidate Chemicals under the DTSC proposal.
Though the addition of microplastics to the Candidate Chemicals List would not in itself result in new regulatory requirements, new obligations would emerge if DTSC proposes and adopts a new Priority Product, which could have a significant impact on manufacturers and businesses who sell their products in California. If you have any questions about how the DTSC proposal may impact your business, please contact:
Tom A. Lewandowski, Ph.D., DABT, ERT, ATS
Principal
tlewandowski@gradientcorp.com
Matthew P. Tymchak, M.S.
Senior Hydrologist
mtymchak@gradientcorp.com
Tim Verslycke, Ph.D.
Principal
tverslycke@gradientcorp.com
Andrew Yeh, Ph.D., DABT
Senior Toxicologist
ayeh@gradientcorp.com
US EPA proposes that any detectable amount of lead in indoor dust poses a hazard and tightens requirements for lead-abatement activities for most pre-1978 housing and child-occupied facilities.
On July 12, US EPA proposed changes to the dust-lead hazard standard (DLHS) and dust-lead clearance level (DLCL) under section 402 of the Toxic Substances Control Act (TSCA). These proposed changes would revise the DLHS, which identifies potentially hazardous concentrations of lead in indoor dust for pre-1978 housing or childcare facilities, to 0 μg/ft2, meaning that any amount of lead detected on floors or window sills would exceed the standard. In addition, the DLCL, which is the acceptable amount of lead that can remain in indoor dust following abatement activities, would be lowered to 3 μg/ft2 (floors), 20 μg/ft2 (window sills), and 25 μg/ft2 (window troughs). A summary of how the proposed changes compare to current dust-lead standards is presented below:
Proposed Changes to Dust-Lead Standards
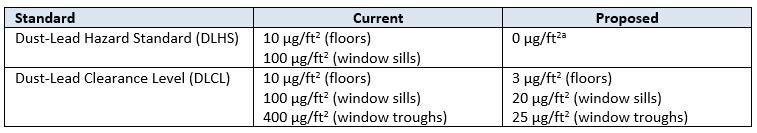
Note: μg/ft2 = Micrograms of Lead Per Square Foot.
(a) As analyzed by any laboratory recognized by US EPA’s National Lead Laboratory Accreditation Program.
Historically, the DLHS and DLCL have been set at the same level; however, in response to a May 2021 court decision, the DLHS was revised to be based solely on health factors, whereas the DLCL can incorporate additional factors (e.g., safety, effectiveness, and reliability). The proposed changes represent the first time these dust-lead standards will be set at different levels. In lowering the DLHS to 0 μg/ft2, the US EPA stated in the proposed rule that “[t]here is no evidence of a threshold below which there are not harmful effects from lead exposure, including neurobehavioral and cognitive effects on children.”
The implementation of the 0 μg/ft2 DLHS is unclear. US EPA noted that the DLHS does not “compel property owners or occupants to evaluate their property for lead-based paint (LBP) hazards nor take control actions.” However, if a lead-abatement activity is performed, then it would be required to meet the DLCLs set forth in the proposed rule.
According to the US EPA, the proposed rule has the potential to impact those in building construction, real estate, child daycare services, elementary and secondary schools, lead abatement, and testing laboratories, among other entities. The US Department of Housing and Urban Development (US HUD) estimates that over 30 million US homes contain lead-based paint.
The proposed rule is still in pre-publication; when it is published in the Federal Register via docket (EPA-HQ-OPPT-2023-0231), US EPA will accept public comments for 60 days. If finalized, the updated DLCLs would go into effect one year after publication of the final rule.
If you have any questions about the proposed dust-lead standards or their implications, please feel free to contact:
Steven Boomhower, Ph.D.
Senior Toxicologist
sboomhower@gradientcorp.com
Rosemary Mattuck, M.S.
Senior Environmental Engineer
rmattuck@gradientcorp.com
Lisa Bailey, Ph.D.
Principal
lbailey@gradientcorp.com
Kurt Herman is a member of Sigma Xi – Scientific and Engineering Honorary, MIT Chapter.
US EPA Proposes New or Strengthened Causal Relationships Between Lead Exposure and Health Effects in Its Updated “Integrated Science Assessment for Lead”
In its recently released draft update to the “Integrative Science Assessment for Lead,” US EPA proposed that scientific evidence now suggests lead exposure causes kidney effects and mortality, among other health effects.
In early 2022, US EPA announced plans to release an update to its “Integrated Science Assessment (ISA) for Lead (Pb),” and recently released the first draft. This updated draft ISA for Pb is part of a broader review of the National Ambient Air Quality Standards (NAAQS) for Pb. In the draft ISA, US EPA concluded that recent scientific evidence supports new, strengthened, or weakened causal relationships between Pb exposure and certain health effects. US EPA evaluates the overall strength of evidence for potential causal relationships between Pb and health effects using a five-level hierarchy, spanning “causal” to “not likely to be a causal relationship.”
A summary of the proposed changes in causal relationships outlined in the draft ISA for Pb, along with those published in the last ISA, is presented in the table below.
In addition, US EPA has released the third volume of its Integrated Review Plan (IRP Volume 3) for the NAAQS for Pb. In the IRP Volume 3, US EPA reaffirmed its conclusion that cognitive effects in children (specifically, intelligence quotient [IQ] decrement) remain to be the most sensitive endpoint on which the primary NAAQS for Pb should be based, as well as any potential changes in the primary standard.
Following review and feedback from the US EPA’s Clean Air Scientific Advisory Committee on June 13-14, 2023, the draft ISA for Pb will be revised; it is anticipated to be released in its final form in spring 2024.
If you have any questions about the updated draft ISA for Pb, the IRP Volume 3, or their implications, please visit our website or contact:
Steven Boomhower, Ph.D.
Senior Toxicologist
sboomhower@gradientcorp.com
Denali Boon, Ph.D., M.P.H.
Senior Epidemiologist
dboon@gradientcorp.com
Topics: Lead, Air Quality, Integrated Science Assessment (ISA), National Ambient Air Quality Standards (NAAQS)
Gradient will present on a panel discussion hosted by Boston Analytical on Navigating Unknown Compounds in Medical and Combination Device E&L Studies, from a Chemistry, Toxicology and Regulatory Standpoint! The panel will include individuals from Boston Analytical, Gradient and Kymanox – See below:
Will Parker, Director of E&L and Nitrosamines Laboratories at Boston Analytical
@Stefanie Johns, PhD, Director, Regulatory Affairs at Kymanox
Isaac Mohar, Ph.D., DABT, Principal Scientist/Toxicologist at Gradient
Click below to register today!
https://lnkd.in/eHetvZFZ
#extractables #leachables #regulatoryguidance #chemistry #toxicology #medicaldevices #combinationdevices #unknowns #unknowncompounds #devicesafety #industryknowledge #stayintheknow